Which statements accurately describe elements check all that apply. – Embarking on an exploration of which statements accurately describe elements, this discourse delves into the fascinating realm of chemistry, unraveling the intricacies of matter and its fundamental building blocks. Through a comprehensive examination of element properties, identification techniques, and their significance in shaping our world, we embark on a journey to decipher the enigmatic nature of elements.
Delving into the heart of the topic, we will meticulously dissect the defining characteristics of elements, scrutinizing their role in the formation of compounds and molecules. By unraveling the methods employed to identify elements, we gain invaluable insights into their unique properties and behaviors.
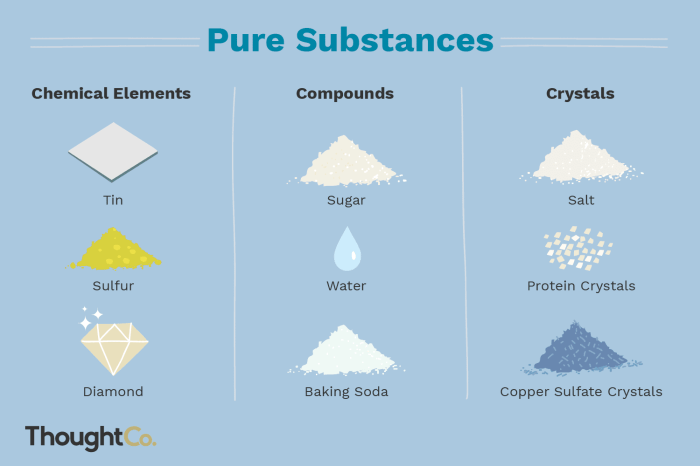
Elements
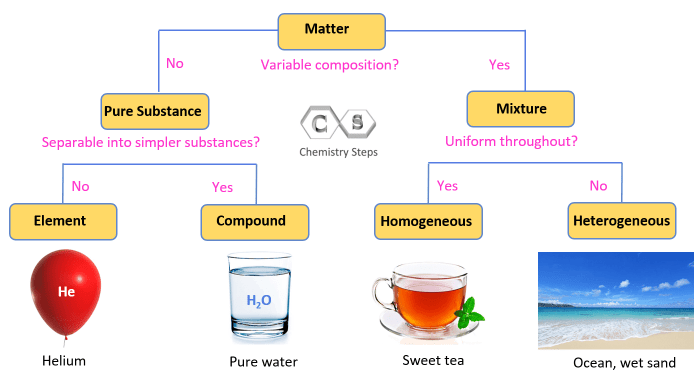
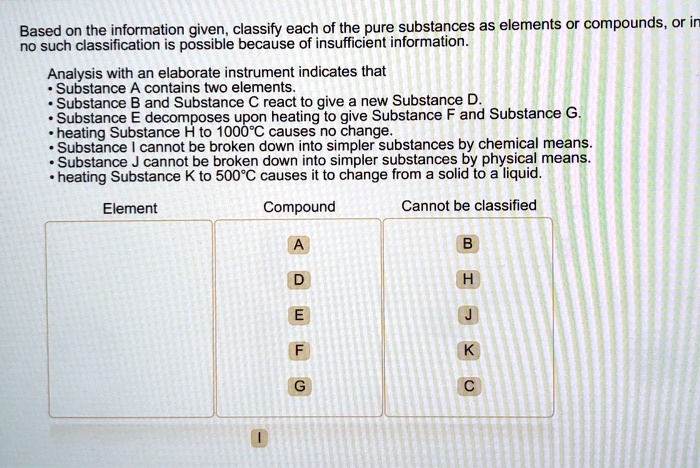
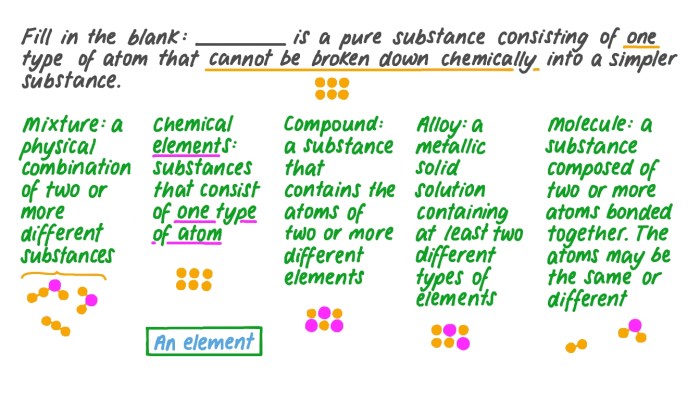
Elements are the fundamental building blocks of matter and the simplest substances that cannot be broken down into simpler substances by chemical means. They are composed of atoms, which are the smallest units of matter that retain the properties of an element.
Elements are identified by their atomic number, which is the number of protons in the nucleus of an atom. There are 118 known elements, of which 94 occur naturally on Earth, while the remaining 24 are synthetic.
Elements play a crucial role in forming compounds and molecules. When two or more elements combine, they form chemical compounds, which are held together by chemical bonds. Molecules are formed when two or more atoms of the same element combine.
Identifying Elements
Elements can be identified using various methods, including spectroscopic analysis and chemical reactions. Spectroscopic analysis involves studying the light emitted or absorbed by an element, as each element has a unique spectral fingerprint. Chemical reactions can also be used to identify elements, as different elements react in characteristic ways with other substances.
Understanding element properties is essential for their identification. Properties such as melting point, boiling point, and reactivity can help differentiate between different elements.
Element Classification
Elements are organized into groups based on their chemical properties, using the periodic table as a reference. The periodic table arranges elements in a way that highlights their similarities and differences. Elements in the same group (vertical column) have similar chemical properties, while elements in the same period (horizontal row) have the same number of electron shells.
The position of an element on the periodic table can provide valuable information about its reactivity. Elements in the same group tend to have similar reactivity, while elements in the same period tend to have different reactivity.
Element Properties
Elements have distinct physical and chemical properties. Physical properties include melting point, boiling point, density, and color. Chemical properties include reactivity, oxidation state, and electronegativity.
These properties influence the behavior and applications of elements. For example, elements with low melting points are used in solders and low-temperature applications, while elements with high boiling points are used in high-temperature applications.
Element Reactivity, Which statements accurately describe elements check all that apply.
Element reactivity refers to the tendency of an element to participate in chemical reactions. Reactivity is determined by several factors, including the element’s atomic number, electron configuration, and electronegativity.
Highly reactive elements, such as sodium and potassium, readily react with other elements to form compounds. Unreactive elements, such as gold and platinum, are less likely to react with other elements.
Element Compounds
Elements form compounds through chemical reactions. When two or more elements combine, they form chemical bonds, which are the forces that hold the atoms together. There are various types of chemical bonds, including ionic bonds, covalent bonds, and metallic bonds.
The type of chemical bond formed between elements determines the properties of the compound. For example, ionic compounds are typically brittle and have high melting points, while covalent compounds are typically soft and have low melting points.
Expert Answers: Which Statements Accurately Describe Elements Check All That Apply.
What are the key characteristics of elements?
Elements are distinguished by their unique atomic number, which determines the number of protons within their nuclei. This atomic number governs the element’s position on the periodic table and influences its chemical properties.
How can elements be identified?
Various techniques are employed to identify elements, including spectroscopic analysis, which examines the element’s light emission or absorption patterns, and chemical reactions, which reveal the element’s reactivity with other substances.
What is the significance of the periodic table in understanding elements?
The periodic table serves as a powerful tool for organizing and classifying elements based on their chemical properties. It allows scientists to predict the behavior and reactivity of elements based on their position within the table.