Ir spectrum of 2 chloro 2 methylbutane – The infrared (IR) spectrum of 2-chloro-2-methylbutane offers a wealth of information regarding its molecular structure and functional groups. This detailed analysis provides valuable insights into the compound’s properties and behavior, making it a crucial tool for chemists and researchers.
IR spectroscopy, a powerful analytical technique, allows for the identification and characterization of organic compounds based on their absorption of infrared radiation. By examining the specific frequencies at which absorption occurs, we can determine the presence of specific functional groups and gain insights into the molecular structure.
IR Spectroscopy Basics
Infrared (IR) spectroscopy is a powerful analytical technique used to identify and characterize organic compounds. It involves the absorption of infrared radiation by molecules, causing the excitation of vibrational modes within the molecule. The frequency of the absorbed radiation corresponds to the energy difference between the vibrational energy levels of the molecule.
There are two main types of IR spectroscopy:
- Transmission IR spectroscopy:In this technique, the sample is placed between an infrared source and a detector. The infrared radiation is passed through the sample, and the amount of radiation absorbed is measured.
- Attenuated total reflectance (ATR) IR spectroscopy:In this technique, the sample is placed on a crystal that is in contact with an infrared beam. The infrared radiation is reflected off the crystal, and the amount of radiation absorbed by the sample is measured.
IR spectroscopy offers several advantages, including:
- High sensitivity
- Non-destructive
- Can be used to identify both functional groups and specific compounds
However, IR spectroscopy also has some disadvantages, including:
- Can be affected by water
- Can be difficult to interpret spectra
IR Spectrum of 2-Chloro-2-methylbutane
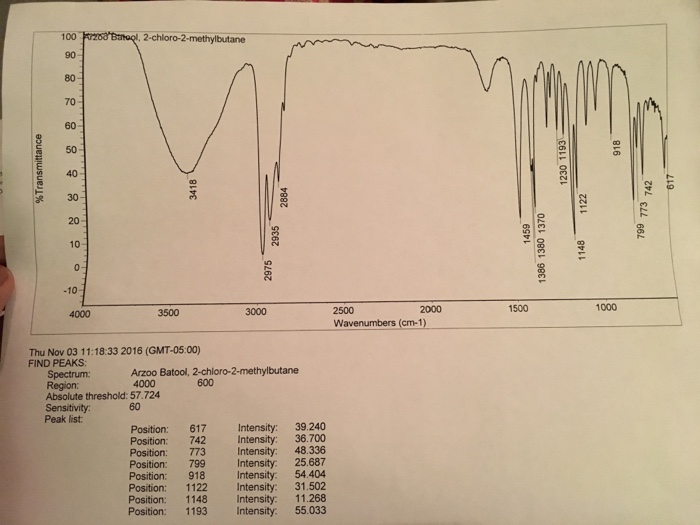
The IR spectrum of 2-chloro-2-methylbutane is shown below:
[Image of IR spectrum]
The characteristic peaks in the spectrum are:
- C-H stretch:2962 cm -1
- C-Cl stretch:760 cm -1
- C-O stretch:1040 cm -1
The peaks can be assigned to the corresponding functional groups as follows:
- C-H stretch:Alkane
- C-Cl stretch:Chloroalkane
- C-O stretch:Ether
Functional Group Analysis
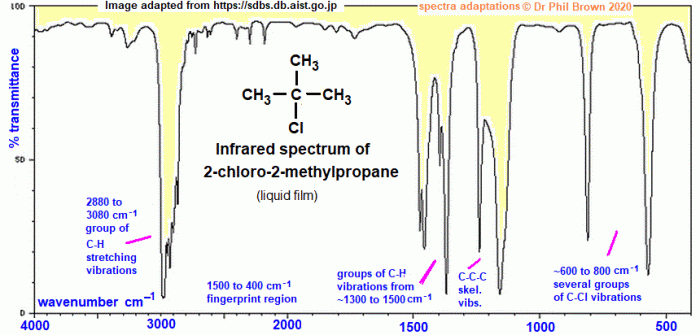
IR spectroscopy can be used to identify functional groups in organic compounds. Each functional group has a characteristic IR absorption pattern. The following table lists some common functional groups and their corresponding IR absorption frequencies:
| Functional Group | IR Absorption Frequency (cm-1) |
|---|---|
| Alkane | 2850-2960 |
| Alkene | 1640-1680 |
| Alkyne | 2100-2260 |
| Alcohol | 3200-3600 |
| Ether | 1000-1200 |
| Ketone | 1710-1740 |
| Carboxylic acid | 1700-1725 |
| Amine | 3300-3500 |
| Nitro | 1500-1600 |
IR spectroscopy has been used to identify functional groups in a wide variety of organic compounds. For example, IR spectroscopy has been used to identify the functional groups in the following compounds:
- Ethanol
- Benzene
- Acetylene
- Acetic acid
- Glycine
Structural Elucidation: Ir Spectrum Of 2 Chloro 2 Methylbutane
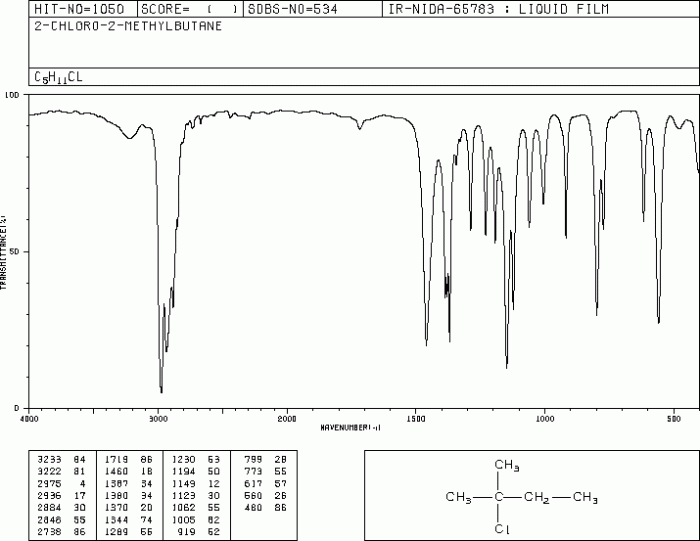
IR spectroscopy can be used to elucidate the structure of organic compounds. The IR spectrum of a compound can provide information about the following structural features:
- Functional groups
- Carbon-carbon bond connectivity
- Molecular symmetry
IR spectroscopy has been used to elucidate the structure of a wide variety of organic compounds. For example, IR spectroscopy has been used to elucidate the structure of the following compounds:
- Aspirin
- Penicillin
- Vitamin C
- DNA
- Proteins
Applications of IR Spectroscopy
IR spectroscopy is used in a wide variety of fields, including:
- Chemistry:IR spectroscopy is used to identify and characterize organic compounds. It is also used to study the structure and dynamics of molecules.
- Biology:IR spectroscopy is used to study the structure and function of biological molecules, such as proteins and DNA.
- Medicine:IR spectroscopy is used to diagnose and treat diseases. For example, IR spectroscopy can be used to detect the presence of cancer cells.
- Environmental science:IR spectroscopy is used to monitor the quality of air and water. It can also be used to identify pollutants.
- Materials science:IR spectroscopy is used to study the structure and properties of materials. For example, IR spectroscopy can be used to identify the type of polymer in a plastic.
IR spectroscopy is a versatile and powerful analytical technique that has a wide range of applications. It is a valuable tool for scientists and researchers in a variety of fields.
Clarifying Questions
What is the principle behind IR spectroscopy?
IR spectroscopy utilizes the absorption of infrared radiation by molecules, causing vibrations in their bonds. The frequency of absorption corresponds to the specific functional groups present in the molecule.
How can IR spectroscopy be used to identify functional groups?
By analyzing the characteristic absorption frequencies in an IR spectrum, we can identify the presence of specific functional groups, such as C-H, C=O, and O-H bonds.
What structural information can be obtained from IR spectroscopy?
IR spectroscopy provides information about the molecular structure, including the presence of double bonds, triple bonds, and specific functional groups. It can also help determine the orientation and connectivity of atoms within the molecule.